FirstHealth Moore Regional Hospital has recently announced that it is one of the first nonacademic hospitals in North Carolina to offer the world’s smallest pacemaker for patients with bradycardia.
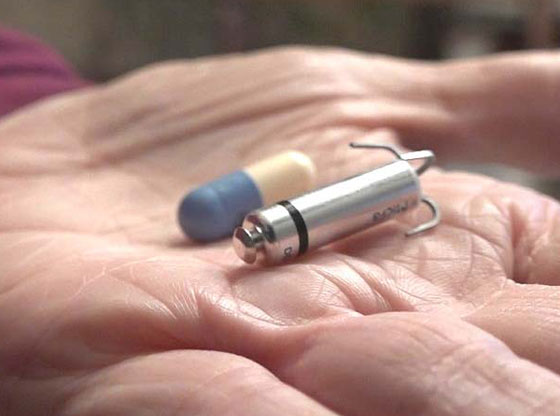
The Micra® Transcatheter Pacing System (TPS) is a new type of heart device that provides patients with the most advanced pacing technology at one-tenth the size of a traditional pacemaker. The first procedure at Moore Regional was performed by Ker Boyce, M.D., FACC, FACP, on March 1, 2018.
Bradycardia is a condition characterized by a slow heartbeat, usually fewer than 60 beats per minute. At this rate, the heart is unable to pump enough oxygen-rich blood to the body during normal activity or exercise, causing dizziness, fatigue, shortness of breath or fainting spells. Pacemakers are the most common way to treat bradycardia to help restore the heart’s normal rhythm and relieve symptoms by sending electrical impulses to the heart to increase the heart rate.
Comparable in size to a large vitamin, physicians at Moore Regional Hospital have elected to use Medtronic’s Micra TPS because unlike traditional pacemakers, the device does not require cardiac wires (leads) or a surgical “pocket” under the skin to deliver a pacing therapy.
Instead, the device is small enough to be delivered through a catheter and implanted directly into the heart with small tines, providing a safe alternative to conventional pacemakers without the complications associated with leads – all while being cosmetically invisible. The Micra TPS is also designed to automatically adjust pacing therapy based on a patient’s activity levels.

Ker Boyce, M.D., FACC, FACP,
“The Micra TPS is the first U.S. Food and Drug Administration (FDA) approved leadless pacemaker,” says Ker Boyce, M.D., FACC, FACP, board certified cardiologist at FirstHealth Moore Regional Hospital. “It is a single chamber ventricular pacemaker, and the device is not for everyone. If you require AV synchrony, biventricular pacing or a defibrillator, you must receive a traditional pacemaker or ICD. However, it is the next technological step in the evolution of pacemakers and I expect to see further developments in the near future.”
The Micra TPS also incorporates a retrieval feature to enable retrieval of the device when possible; however, the device is designed to be left in the body. For patients who need more than one such device over their lifetime, the miniaturized Micra TPS was designed with a unique feature that enables it to be permanently turned off so it can remain in the body and a new device can be implanted without risk of electrical interaction.
The Micra TPS is the first and only transcatheter pacing system to be approved for both 1.5 and 3 Tesla (T) full-body magnetic resonance imaging (MRI) scans and is designed to allow patients to be followed by their physicians and send data remotely via the Medtronic CareLink® Network.
The Micra TPS was approved by the FDA in April 2016, and has been granted Medicare reimbursement, allowing broad patient access to the novel pacing technology.
For information on FirstHealth’s heart services visit www.firsthealth.org/heart or call (800) 213-3284.